We Beasties has moved!
We’ve moved to ScienceBlogs! Come check us out at our new digs 🙂
population genetics, evolution, and ocean ecosystems
I was trained as an Environmental Scientist long before I was at all interested in Microbes. So, I get excited when I come across microbial studies that are environmentally relevant. I get particularly nerd-cited when these studies take place in the ocean. A paper published in PNAS last week describes identifies what may be the key environmental factor distinguishing the evolution of microbial populations in the North Atlantic and North Pacific sub-tropical gyres.
The finding that populations of the abundant, widespread, and relatively well studied marine microbes Prochlorococcus and Pelagibacter (both) in an Atlantic study site had much higher frequencies of genes related to phosphorus (P) acquisition and metabolism than similar organisms in a Pacific study site was no surprise to the researchers. What was shocking was that virtually all of the genes with significantly different frequencies in the two sites were related to P use or uptake. This implies that reduced P concentrations in the Atlantic (relative to the Pacific) is the primary, and possibly sole, environmental factor driving the evolution of the P limited population. Environmental complexity generally prevents such complete correlations in studies comparing distant environments.
The methods in this study involved sampling water at three different depths at the two sites, isolating and culturing microbes of the two types and they sequencing the genomic DNA each population. Then they analyzed the variety in the sequences, and compared them to previously sequenced lab strains of each type, looking for genes that were present in some, but not all of microbes in the population. Most of these differences were due to random variation and neutral (not evolutionarily advantageous) evolutionary processes (genes are often passed around through horizontal gene transfer, but they not always advantageous). The genes of interest were those that were more abundant (statistically speaking) in one site or the other, because that indicates that that gene is conferring some evolutionary advantage in that environment, but not in the other. In Prochlorococcus, there were 29 such genes, and nearly all of them were more abundant in the Atlantic site, and were phosphorus-related. This pattern was confirmed with Pelagibacter. The conclusion is strengthened by the fact that these two types organisms are very different. Prochlorococcus is a photosynthetic cyanobacteria, where as Pelagibacter is a heterotroph
Microbial ecology is a relatively young science, and very little ecological theory has been tested with microbial populations. Studies like this one allow scientists to make predictions (that future work can support or contradict) about how evolution is working in microbial populations in natural environments. These types of studies on marine microbes are especially important because we know so little about these communities, and many of them are beginning to deal with changing environmental conditions. The paper concludes with the following statement.
In this way, population genomics of ocean microbes not only is a powerful tool for diagnosing environmental change, but also can illuminate the fundamental evolutionary processes underlying biological organization.
Coleman ML, & Chisholm SW (2010). Ecosystem-specific selection pressures revealed through comparative population genomics. Proceedings of the National Academy of Sciences of the United States of America PMID: 20937887
Everything’s contaminated (redux)
Have you noticed the recent spate of people coming down with terrifying bacterial infections contracted at Apple stores? Yeah, me neither. Still:
A leading Australian expert in infectious diseases says people who use display iPads and iPhones at Apple stores are risking serious infections and the company should do more to maintain hygiene[…]
“You wouldn’t have hundreds of people using the same glass or cup, but theoretically if hundreds of people share the same keyboard or touch pad, then effectively that’s what you’re doing,” Collignon said in a phone interview.
This, following an “investigation” in June by the New York Daily News that showed high levels of various germs on various gadgets in Apple stores. “Ew,” goes one quote from a random person coming out of the store. So should we all start wearing surgical gloves and gas masks when going to the Apple store?
As I’ve said before – the more shocking result would be if they HADN’T found any bacteria. Everything’s contaminated, period. Maybe since the Apple store seems so pristine and sterile, it’s rife for this kind of criticism. But seriously, it’s retarded. Swab any door handle, kitchen countertop, or human forearm and you’d likely be able to write the same sort of scare story. But you don’t generally see people walking around on perpetual regimins of broad-spectrum antibiotics.
A separate test of a sample of 30 mobile phones, conducted by a hygiene expert at Britain’s Which? magazine, found that the average handset carries 18 times more potentially harmful germs than a flush handle in a men’s toilet.
Well, no shit (pun only partially intended). Any half-way decent bathroom is cleaned at least once a day with some serious de-sterilizing cleansers. Unless some guy crapped on his hand and then flushed the toilet just before this test was taken – I would imagine the flush handle to be one of the cleanest locations you could survey.
So relax, play with the pretty gadgets, and if you’re really scared, just make sure you use a Dyson hand dryer after you wash your hands.
The Antibiotics of Color
Bacterial infections are problematic for any creature, even the high-flying ones. A new paper in Biology Letters shows that the colorful pigments of parrots may play a role in bacterial resistance:
We exposed a variety of colourful parrot feathers to feather-degrading Bacillus licheniformis and found that feathers with red psittacofulvins degraded at about the same rate as those with melanin and more slowly than white feathers, which lack pigments. Blue feathers, in which colour is based on the microstructural arrangement of keratin, air and melanin granules, and green feathers, which combine structural blue with yellow psittacofulvins, degraded at a rate similar to that of red and black feathers.
The paper is pretty straightforward – it’s all right there in the abstract – but the claims they make about importance aren’t really backed up by any data.
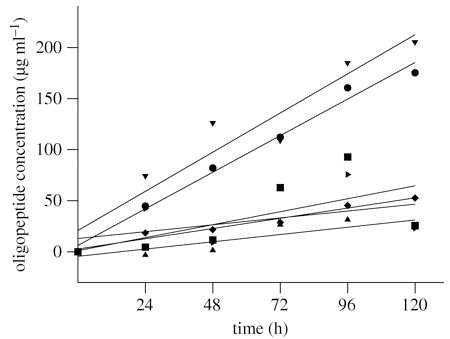 Here, they’re plotting the concentration of certain bacterial bi-products over time when an initial inoculation of Bacillus licheniformis is allowed to grow on different colored feathers (the different shapes correspond to different pigments present). On the one hand, it seems pretty clear that white feathers (the top line – upside-down triangles) are very permissive to bacterial growth, but black, red and blue feathers (the bottom 4 lines) are restrictive.
Here, they’re plotting the concentration of certain bacterial bi-products over time when an initial inoculation of Bacillus licheniformis is allowed to grow on different colored feathers (the different shapes correspond to different pigments present). On the one hand, it seems pretty clear that white feathers (the top line – upside-down triangles) are very permissive to bacterial growth, but black, red and blue feathers (the bottom 4 lines) are restrictive.
On the other hand, the claim that these pigments evolved for the purpose of blocking bacterial growth is unsubstantiated. It seems totally plausible, don’t get me wrong, but all birds have feather-degrading bacteria, and many have evolved bright pigments that don’t necessarily have this anti-microbial activity. It might be interesting to follow this up with an epidemiological study to see if these parrots in the wild are less prone to bacterial infection in order to make a more direct causal link.
Burtt EH Jr, Schroeder MR, Smith LA, Sroka JE, & McGraw KJ (2010). Colourful parrot feathers resist bacterial degradation. Biology letters PMID: 20926430
Ig Nobel awards go microbial
This post was team co-written by Dipti and Heather
The well-renowned Nobel Prize speaks for its own worth. Scientists, activists, economists, politicians and others who have made an exceptional mark in their respective fields are bestowed this great and rare honor.
What happens to the quirkier researchers though? What honor can one hope to receive in recognition for making bras that can act as emergency gas masks, proving that a digital rectal massage can cure long term hiccups, studying the intricacies of bat fellatio or showing how magnets can be used to levitate frogs?. They win the equally quirky Ig Nobel Prize – the lesser known, far more entertaining cousin. This prize is awarded for “achievements that first make people laugh, and then make them think”.
Each year the Ig Nobel awards are given out in Harvard’s Sanders Theater honoring the most outstanding “Improbable Research” of the year in various categories. For example this year’s Ig Nobel Peace Prize this year was given to Richard Stephens, John Atkins, and Andrew Kingston of Keele University, UK, for confirming the widely held belief that swearing actually relieves pain, while the engineering prize was awarded to Karina Acevedo-Whitehouse and Agnes Rocha-Gosselin of the Zoological Society of London, UK, and Diane Gendron of Instituto Politecnico Nacional, Baja California Sur, Mexico, for perfecting a method to collect whale snot, using a remote-control helicopter.
Actual Nobel Laureates hand out these awards and quite a show is put on about the whole thing. There is music, drama, absurd costumes, audience participation, science demonstrations, and it all makes for a very entertaining show.
Each year a theme is chosen that comes up repeatedly throughout the night. Evelyn Evelyn – a band starring conjoint twins, of which one is Amanda Palmer – kickstarted the award ceremony with an aptly titled song called Bacteria Bacteria to announce this microbial years theme – bacteria! Two of the contributors to this blog were in attendance and even got to march in at the beginning dressed as bacteria representing Harvard’s Microbial Sciences Initiative (see photo above). Bacteria did get a bit of a bad rap during the show… there was much attention paid to the pathogenic bacteria, but given how much a tooth can ache, we suppose that is justified.
The microbial highlight of the evening was, in fact, a 3 act opera that took place on the surface of a woman’s tooth.
If nothing else, these awards are definitive proof that many (We might even say most) top-notch scientists have a sense of humor, albeit a quirky one. Please note that quirky, improbably science can be serious and noteworthy simultaneously. One Ig Nobel award winner (from 2000) won a Nobel prize in Physics this year and another the Macarthur genius grant last year. The Ig Nobel is our favorite science prize precisely because it recognizes quirky but GOOD science, doesn’t take itself seriously, and has a soft spot for bacteria.
More on protein folding and video games
A while ago, I wrote about an awesome paper in Nature that had people play a video game to learn about protein folding. Well, I was just catching up on a back-log of podcasts, and in a Meet the Scientist podcast last month, Carl Zimmer interviews David Baker, the lead author of that study.
Carl Zimmer is easily my favorite science journalist, and if you don’t read his stuff, you should. The podcast isn’t half-bad either.
Implications of the Immune response
I started writing this post before I read ERV dissecting some “the immune system is perfect” BS. Go read hers, then come back if you want more.
Now that I’ve gone through the basics of a typical immune response, I think it’s necessary to point out some of its many flaws. In many of the immunology courses I’ve taken, the mammalian immune system is presented almost as the pinnacle of evolution, but it is far from perfect. In fact, in many ways, we might be better off if it had never evolved at all.
First up – Autoimmunity. T-cells and B-cells generate random receptors that can in principal see any molecular shape, and that includes shapes that our own body produces. Intrinsically, T and B cells (collectively called lymphocytes) have no way of knowing if their receptor sees some virulent strain of E. coli or they myelin sheath of your own neurons. To counteract this problem, we have evolved elaborate mechanisms to promote immune tolerance of our own tissues. Developing lymphocytes are programed to self-destruct if their receptor binds something early on (before they are likely to have seen a real pathogen), and they get turned off if they bind something in the absence of a danger signal (usually provided by a pattern recognition receptor). There are also regulatory cells flying around the blood stream, tamping down runaway signals and trying to keep them quiescent.
But these mechanisms often break down, and we have diseases like multiple sclerosis, type-1 diabetes, rheumatoid arthritis, Crohn’s disease, Lupus, etc. In addition, there are so-called hyper-inflammatory disorders in which the immune system over-reacts to harmless molecules, yielding the wonderful trifecta of allergies, asthma, and IBD.
“But surely,” you say, “those are rare side-effects of a system that, on balance, is protective.” I’m not so sure. The second obvious flaw with our immune system is that it doesn’t actually protect us from the virulence of pathogens. This statement seems deeply counterintuitive (especially coming from an immunologist), but hear me out. How many of you readers have never gotten sick? I feel pretty confident saying that no one raised their hand. “But without an immune system, that pathogen would have killed me,” you say, and this is sort of true. Certainly, people with compromised immune systems are at a much higher risk for death from fairly routine infections, but the real question is, if no one had an immune system, what would be the outcome?
To understand this point, I think it helps to look at this from the pathogens’ perspective. What is the goal of a rhinovirus (that might cause a cold), or Plasmodium falciparum (which causes malaria), or Bacillus anthracis (better known as anthrax)? The goal is not (necessarily) to kill you. The goal is to maximize replication and transmission. And depending on the mode of transmission, there are different levels of virulence (ability to make you sick) that are more beneficial.
Transmission of a cold virus occurs by person-to-person contact. You’ll infect far more people if you’re just sick enough to still go to work or go to buy groceries. If that rhinovirus made you as sick as malaria, you’d never infect anyone. Plasmodium falciparum, on the other hand, has mosquito vectors that carry it around, and you’re actually less of a threat to the mosquitos if you’re incapacitated. It doesn’t want to kill you, because then the parasite will die as well. People do die from malaria, but that’s an unintended side effect, and in any case those people have probably already fed many mosquitos and passed the disease on. B. anthracis doesn’t care if you die, but that’s because its method of transmission doesn’t depend on its host living. It just wants to replicate as much and as quickly as possible, devouring whatever nutrients it can get its hands on. If and when the host dies, Bacillus can form spores, which are extraordinarily hearty. They can withstand all kinds of extremes (even autoclaves – the industrial sterilizers used in labs and hospitals won’t kill Bacillus spores), and can lay dormant for decades or even centuries, just waiting for a new hapless host to wander along.
This whole idea is called virulence theory, and if you think about it, the best way to guess how virulent a pathogen will be is to look at its method of transmission. Of course, there are exceptions, but the exceptions aren’t particularly successful pathogens. Several hemoragic fever viruses (think Ebola) spring up now and again and wipe out whole villages, but then they die off because they incapacitated and then killed their entire host supply too rapidly to make it to a neighboring village. But what does all this have to do with the immune system?
Pathogens have evolved to expect an immune system, and they’ve gauged their virulence accordingly. That’s why an AIDS patient can die from a normally harmless bacterial infection – those bacteria expect push-back from an immune system that isn’t there. But if we had never evolved immune systems, the pathogens wouldn’t have evolved all of those ways around it, and we would all expend far less energy and arrive at the same outcome. This is a classic example of an Red Queen evolution – an arms race between competitors. And you can see it everywhere in life, from cheetahs and antelopes to hundred-foot tall redwood trees. And my larger point is not really that the immune system is useless – clearly it was beneficial enough to our ancestors to be worth the price of energy expenditure and potential autoimmunity. The larger point is that when I write (as I often do) about the ways that pathogens get around our high and mighty immune system, remember that these evasion strategies are the rule, not the exception.
Walking Bacteria – And some weighty researcher cajones
![]() Most papers I read these days are long. Nature and Science papers tend to have 3-4 figures (Cell and Immunity papers can be twice that), tons of supplementary data and are at least a couple pages of dense, science-speak prose. I think I once read a paper (from like 20 years ago) that had a gene sequence as figure 1, a hand-drawn model for figure 2 and one figure of functional data, and I thought that was sparse.
Most papers I read these days are long. Nature and Science papers tend to have 3-4 figures (Cell and Immunity papers can be twice that), tons of supplementary data and are at least a couple pages of dense, science-speak prose. I think I once read a paper (from like 20 years ago) that had a gene sequence as figure 1, a hand-drawn model for figure 2 and one figure of functional data, and I thought that was sparse.
So imagine my surprise when I stumbled on this new paper. One figure. Less than 500 words. And it’s about bacteria that seem to get up on their legs (wait, bacteria have legs?!?!). Published in Science – one of the most prestigious science journals in the world. Anyone that is willing to submit a 1 figure paper (not to mention get it accepted) in Science with a sentence like
Bacteria stood upright and “walked” […]
in the abstract is either extremely clever, or extremely ballsy (or a healthy combination of both).
Here’s what they did: they took pictures of huge numbers of Pseudomonas aeruginosa at the surface of biofilms and then used computer software to analyze how individual bacteria were behaving. They noticed that a large number of bacteria near the surface appeared to lift up into a vertical orientation, then walk along the surface of the biofilm on little appendages called type-IV pilli (TFP). I’ve mentioned biofilms before, but the easiest way to think of them is as a bacterial community. Mostly we think of bacteria as single-celled individuals, but biofilm-forming bugs can achieve a measure of cooperation, and the formation of biofilms is a requirement for a lot of bacterial pathogens (like Pseudomonas) to actually cause disease.
The TFP’s were always known to be used for locomotion, like the propellers of a boat. Indeed, when these bugs were in a horizontal orientation, they could crawl in straight lines for long distances. But in the standing orientation, the pilli seemed to act like legs for the bacteria to scuttle along at a faster rate, though they seemed less directional:
Each mechanism confers advantages for surface exploration[…] Crawling enabled directional motion; walking enabled rapid local exploration.
In part A of this figure, red represents the “walking” orientation, and is a pictorial representation of the motion of individual bacteria – each line represents a single cell’s motion over time. Comparing those lines to the blue ones, you can see that the crawling orientation tends to be much longer and much straighter.
Part B is just quantifying (putting into numbers) what is represented in A. They actually analyzed about 70,000 individual bugs, and if you look at the axis labeled “L,” it shows the distance that each individual traveled, and you can pretty clearly see that the red guys all cluster in the much shorter distances.
They also mention some observations about the cell division behaviors – the TFP seem to be important for daughter cells to move away from each other after they divide – but this seems like more of an appeal to increase the relevancy of the paper. As I said before, it was known for a while that TFP were required for movement, and none of the data presented demonstrates that this walking movement is necessary. In fact, they say
daughters left the division site by detaching, walking, or crawling
It’s just that TFP are required for all of these events.
Understanding he way that biofilms affect the life cycle of bacteria is crucial to understand the role of biofilms in disease, but the data presented here is really just observation. It doesn’t provide any mechanistic insight, though it will hopefully lead the way to more detailed understanding of how and why this is important. On the other hand, it clearly impressed the editors of Science enough to get included.
Gibiansky, M., Conrad, J., Jin, F., Gordon, V., Motto, D., Mathewson, M., Stopka, W., Zelasko, D., Shrout, J., & Wong, G. (2010). Bacteria Use Type IV Pili to Walk Upright and Detach from Surfaces Science, 330 (6001), 197-197 DOI: 10.1126/science.1194238
Cooperation in Fungal Spores
Suppose you were a microscopic fungal spore, and your success was dependent upon making your way to some far off place away from your parent organism, to begin a new life all your own. Maybe you are a pathogen (such as Sclerotinia sclerotiorum) and can only survive if you go off on your own and find a new host to infect. What if you could coordinate with thousands of other spores like you, and in one synchronized ejection effort you could create an air current that would take many of you upward into the flowing air above? Turns out if you belonged to certain fungi, you could do just that. As amazing as microbes are, we don’t often think of them as having behaviors that are coordinated to the second and visible to the naked eye, but some of them do! Check out the embedded video (courtesy of New Scientist) below for an awesome example of this recently investigated phenomena.
The authors of this new study published in the Proceedings of the National Academy of Sciences (PNAS) entitled “Dispersal of fungal spores on a cooperatively generated wind” state the following:
“Here we show that by synchronizing the ejection of thousands of spores, these fungi create a flow of air that carries spores through the nearly still air surrounding the apothecium, around intervening obstacles, and to atmospheric currents and new infection sites.”
These authors come from mathematics, engineering and biology departments, and as you might expect, they used multiple lines of inquiry in this paper. Additionally, they investigated several different species of fungi that eject spores in a coordinate way, and found similar patterns in how they coordinate. The methods included high-speed photography, complex mathematical modeling, laboratory manipulations, and applying ecological theory to their data and models. It turns out that the range of a cooperating spore can be 20 times greater than a spore that ejects on its own. However, it doesn’t benefit all the spores equally. If you are too early or too late in ejecting you don’t catch the ride, and it take many spores to initially generate the flow that triggers all the others, and those initial launchers don’t get much of a ride themselves. In the end, the end result is that some of the spores get jettisoned up into the air, and end up much further away than they would have without the coordinated activity. Apparently these jets of fungal spores can even move around obstacles. This is certainly an important piece of fungal dispersal ecology. In very practical financial terms this study is important for agriculture, since some of the species engaging in this dispersal behavior are important plant pathogens, and understanding that dispersal could help prevent crop infection.
The extent to which microbes in the environment are able to disperse is a huge factor in their geographic arrangement. Here I group bacteria and archaea together with fungal spores because their size means their dispersal is fundamentally different from larger organisms. Microbial dispersal is something that is difficult to study, and, therefore, not well understood. Better information about of how these organisms get around, and how far they can travel will come out of more interdisciplinary studies such as this one, and is needed in order to understand the ecology of microbes.
Roper M, Seminara A, Bandi MM, Cobb A, Dillard HR, & Pringle A (2010). Dispersal of fungal spores on a cooperatively generated wind. Proceedings of the National Academy of Sciences of the United States of America PMID: 20880834
Immune response from start to finish: Part 3
[I’ve been hooked on the immune system since I was a kid and my dad showed me electron micrographs of macrophages eating bacteria in Scientific American. Now that I’m in graduate school studying immunology, and macrophages in particular, my dad asked if I could give a play-by-play of an immune response. Here you go Dad:]
Part 3: Immune memory
Towards the end of the 18th century, Edward Jenner did an experiment. It had long been known that people who had been infected with smallpox, if they managed to survive (no easy feat), would be resistant to further infection. People would even give small inocula of smallpox to healthy people in an effort to prevent a more serious infection (though this wasn’t very controlled and would often lead to serious illness and death). But there was also anecdotal evidence that milkmaids, who were often afflicted by the much milder disease cowpox, were also resistant to smallpox. So, Jenner devised the hypothesis that cowpox was close enough to smallpox that it would teach the body how to fight both. And he tested it – by injecting James Fipps (the 8 year old son of his gardener) with puss from a cowpox sore. Unsurprisingly, the kid got cowpox, and when the infection cleared, Jenner then injected the same boy (why this kid didn’t run screaming, I will never understand) with puss from someone with smallpox. Magically, the boy did not get small-pox. Thus, Jenner is credited with devising the first vaccine. In fact, the name vaccine comes from “vacca,” the latin word for cow.
Even with all the “controversy” about vaccines, the fact is that they work. One of the benefits of being a chordate is that we have an adaptive immune system, and that branch of the immune system remembers. In the last part, I talked about the T-cells and B-cells of the adaptive immune system. These cells have special, randomized receptors, and each individual cell recognizes something unique. During the course of your life, you’ll make hundreds of billions of different T cell and B-cell receptors, and most of them will never be used. But during an infection, some of the T and B cells will respond, and during that response, they will replicate. Most of the daughter cells will become effectors, and do all the disease-fighting things I talked about before, but a small percentage will become memory cells.
At some point, all of those effector cells have to die off – if they didn’t, after a couple times getting a cold, you’d end up being a giant lymph node. But memory cells know they’re important, and can survive for years or even decades. Because they were activated in the presence of an infection, they can be sure that their receptor recognized something foreign that is potentially dangerous. And if that something rears its head again, the memory cell can rapidly proliferate and produce new effector cells, all without waiting for a dendritic cell to say that it’s ok. In addition, memory B-cells can continue to secrete antibodies, which patrol your bloodstream, just waiting to encounter that pathogen once again.
This last bit is what makes vaccines possible. When Jenner infected James with cowpox, the boy’s immune system responded. Dendritic cells from his skin grabbed bits of the cowpox virus and brought them to lymph nodes to show to T and B cells. Some of those T and B cells had receptors that could recognize those bits, and they expanded and differentiated to run off and battle the infection. Meanwhile, some of them held back and turned into memory cells, and the memory B cells in particular continued to churn out cowpox-specific antibodies. Weeks later, when Jenner inoculated him with smallpox, there were already millions of cowpox antibodies flying around his bloodstream. Since smallpox is closely related to cowpox, many of those antibodies could recognize the virus particles and bind to them, preventing them from infecting any of the boy’s cells. If any viruses slipped by and actually infected a cell, the memory T cells would be alerted and blast the infected cell before it could make many new viruses. And if that cell did manage to make new viruses, those new viruses would also have to get by the antibody wall.
That’s the immune response in a nutshell. To recap: Innate immunity tags and bags most things that get past your barriers, then the adaptive immune response picks up the stuff that gets through, and remembers what infected you so that it can respond better the next time. That’s how I learned it in my undergraduate immunology class. That’s how it’s being taught to the undergrads I’m teaching now. Simple right?
Well, not so fast. If it’s that simple, why don’t we have vaccines against the legions of bacterial infections that debilitate or kill people every year (not to mention HIV), and why do we need a new flu vaccine every year? Why are over 90% of adults chronically infected with various strains of herpes virus? And why are there billions of dollars to be made in drugs that slow the immune system down? I’ll talk about some of the nuance and the complications of our sophisticated immune system next. Stay tuned.
Immune response from start to finish series
Part 1: Invasion and detection: Innate immunity
Part 2: T-cells, B-cells and adaptive immunity
Part 3: Immune Memory (current)


